Background: The Phase II CAVALLI study assessed the B-cell lymphoma 2 (BCL-2) inhibitor venetoclax (Ven; 800mg for 10 days [Ds]) + rituximab (R), cyclophosphamide (C), doxorubicin (H), vincristine (O) and prednisolone (P; R-CHOP), for first line treatment of diffuse large B-cell lymphoma (DLBCL). The end of treatment (EOT) complete response (CR) rate was 64% in patients (pts) with BCL-2 overexpression by immunohistochemistry (BCL-2 IHC+); there was a 2-year progression-free survival (PFS) benefit compared with R-CHOP (GOYA trial; 78% vs 62%); but a higher incidence of Grade (Gr) 3/4 adverse events (AEs; 86% vs 66%, respectively; Morschhauser et al. Blood 2021).
In the Phase III POLARIX study, polatuzumab vedotin (Pola)-R-CHP had a prolonged PFS vs R-CHOP, establishing Pola-R-CHP as standard of care for untreated DLBCL (Tilly et al. N Engl J Med 2022; Morschhauser et al. EHA 2022). Thus, we explored whether adding Ven to Pola-R-CHP could further improve outcomes in BCL-2 IHC+ DLBCL. Here, we report early safety and efficacy results from a Phase Ib study (BO42203; NCT04790903), evaluating the optimal dose/schedule of Ven+Pola-R-CHP.
Methods: BO42203 is an ongoing open-label, multicenter study of pts with untreated BCL-2 IHC+ DLBCL (including Grade 3b follicular lymphoma). Pts enrolled had an International Prognostic Index (IPI) of 2-5, and BCL-2 IHC+ defined as ≥50% expression (by local pathology).
The primary endpoint is to determine the recommended Phase II dose (RP2D) for Ven+Pola R-CHP based on the rate of dose-limiting toxicity (DLT) during the first 2 cycles (42 Ds), with tolerability assessed by dose modifications/delays and discontinuation. Secondary endpoints include percentage of pts with AEs, and PET-based response rates/duration.
Pts are enrolled in 5 cohorts (n=10 pts each). Safety data are reviewed by an internal monitoring committee (IMC) who can alter the Ven dose/schedule for the next cohort. Pts receive 6 21-D cycles of treatment. Pola-R-CHP is administered on D1 of each cycle at the following doses: Pola 1.8mg/kg, R 375mg/m 2, C 750mg/m 2, H 50mg/m 2, and P 100mg/D for 5 Ds. All pts in Cohort 1 were assigned to Ven 800mg/D for 5 Ds/cycle; doses start on D4 of Cycle 1 and D1 of subsequent cycles (Schedule A) with optional escalation to 10 Ds/cycle from Cohort 2 onwards (Schedule B), depending on IMC assessment.
Results: At the time of analysis (data cutoff: May 2, 2023), 4 cohorts were enrolled (n=40). At baseline, the median age was 64.0 years, 14 (35.0%) pts were female, and 5 (12.5%) had an Eastern Cooperative Oncology Group performance status of 2. Thirty-six (90.0%) pts had Ann Arbor Stage III-IV; 30 (75.0%) had an IPI score of ≥3; and 10 (25.0%) had poor risk cytogenetics (double/triple hit lymphoma [DHL/THL]).
Thirty-eight pts have completed the DLT period; 2 pts withdrew prior to this due to meningitis and a COVID-related AE. No DLTs have been observed. Gr ≥3 myelosuppression was observed in Cohort 1 after the DLT period: neutropenia (n=5 pts [50.0%]), neutrophil count decreased (n=2 [20.0%]), and febrile neutropenia (n=1 [10.0%]). Hence, after IMC review of Cohorts 1-3 it was decided that Schedule A will be maintained/received by all future pts, as the benefit-risk of increasing the Ven schedule may not be favorable.
Safety and efficacy analyses were performed on Cohorts 1-3 (n=30) as they have reached the EOT. All pts had ≥1 AE; 21 (70.0%) and 11 (36.7%) pts had at least one Gr ≥3 AE ( Table) and serious AE (SAE), respectively; 2 (6.7%) pts died due to treatment-related SAEs (investigator-assessed; sudden cardiac death [related to Ven] and sepsis [related to Ven+Pola-R-CHP]). AEs leading to dose modification/delay of any drug occurred in 11 (36.7%; 23 events) pts; 21 (91.3%) AEs led to Ven modification in 10 pts (33.3%); and 3 (10.0%) pts had an AE that led to discontinuation of any study drug.
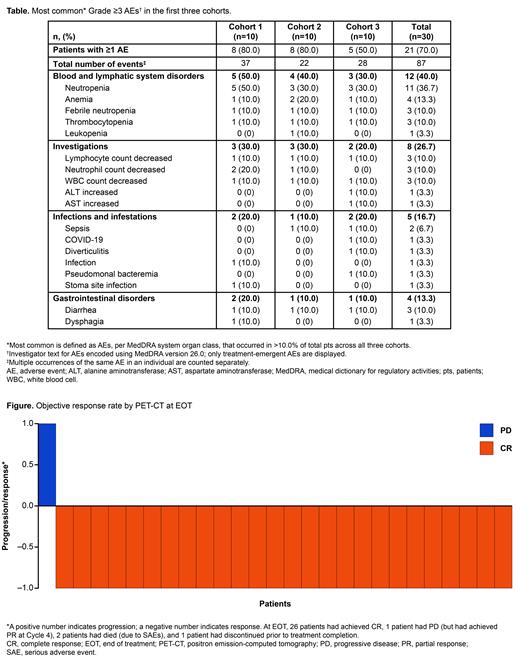
Response was evaluated in 30 pts, with an EOT PET-CT based objective response rate and CR of 86.7% (n=26; including all pts with DHL/THL [n=8; CR: 100%]); 1 pt had progressive disease [PD], and 3 were not assessed due to death (n=2) and early discontinuation (n=1; Figure). Three (10.0%) pts died due to PD.
Conclusions: Ven 800mg/D for 5 Ds/cycle + Pola-R-CHP has been determined as the RP2D; early results show acceptable safety and promising efficacy for untreated BCL-2 IHC+ DLBCL. High CR rates were observed across all cohorts at EOT, including pts with DHL/THL. Updated results, including circulating tumor DNA, will be presented.
OffLabel Disclosure:
Zelenetz:Janssen Pharmaceuticals: Consultancy, Honoraria; None other than mutual funds (401K): Current equity holder in publicly-traded company; Pharmacyclics: Consultancy, Honoraria; SAB: Membership on an entity's Board of Directors or advisory committees; Lymphoma Research Foundation: Membership on an entity's Board of Directors or advisory committees; MEI Pharma Inc: Consultancy, Honoraria, Research Funding; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Abbvie: Research Funding; Gilead: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria. Diefenbach:Gillead: Current equity holder in publicly-traded company; OverT Therapeutics: Current equity holder in private company; F. Hoffmann-La Roche Ltd/Genentech, Inc., BMS, Merck, Abbvie, Novartis, Celgene, Cargo, Nekktar: Research Funding; Genmab, Abbvie, Regeneron, F. Hoffmann-La Roche Ltd/Genentech, Inc., Seattle Genetics, Merck: Membership on an entity's Board of Directors or advisory committees. Herbaux:AbbVie, F. Hoffmann-La Roche Ltd, AstraZeneca, Janssen: Honoraria; AbbVie, Takeda: Research Funding; AbbVie, F. Hoffmann-La Roche Ltd, AstraZeneca, Janssen: Consultancy; Physician and professor of Hematology at academic center (CHU Montpellier France): Current Employment. Tani:Abbvie, Jansen-Cilag, Incyte: Membership on an entity's Board of Directors or advisory committees. Houot:Kite/Gilead, Novartis, Bristol-Myers Squibb/Celgene, ADC Therapeutics, Incyte, Miltenyi: Consultancy; Kite/Gilead, Novartis, Incyte, Janssen, MSD, Takeda, F. Hoffmann-La Roche Ltd: Honoraria. Bastos-Oreiro:SEHH, AMHH: Membership on an entity's Board of Directors or advisory committees; BMS, Kite, Novartis, F. Hoffmann-La Roche Ltd, Incyte, Abbvie: Honoraria, Speakers Bureau; Incyte, Kite: Consultancy; F. Hoffmann-La Roche Ltd, Kite, SEHH, AMHH: Research Funding; Gregorio Maranon Hospital: Current Employment, Membership on an entity's Board of Directors or advisory committees. Tilly:F. Hoffmann-La Roche Ltd: Honoraria, Research Funding; BMS, F. Hoffmann-La Roche Ltd, ADC therapeutics: Membership on an entity's Board of Directors or advisory committees. Thieblemont:Cellectis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding; Kite: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Kyte, Gilead, Novartis, BMS, Abbvie, F. Hoffmann-La Roche Ltd, Amgen: Honoraria; Janssen: Honoraria, Other: Travel Expenses; BMS/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding; Gilead Sciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Hospira: Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Honoraria; Paris University, Assistance Publique, hopitaux de Paris (APHP): Current Employment; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Troy-Barnes:F. Hoffmann-La Roche Products Ltd: Current Employment; University College London Hospitals NHS Foundation Trust, North Middlesex University Hospital NHS Trust: Ended employment in the past 24 months; Whittington Health NHS Trust (honorary contract): Honoraria. Olivieri:F. Hoffmann-La Roche Ltd: Current Employment, Current equity holder in private company. Kesavan:F. Hoffmann-La Roche Ltd: Current Employment; Oxford university hospitals NHS Trust: Ended employment in the past 24 months. Kanwar:Viatris: Ended employment in the past 24 months; F. Hoffmann-La Roche Ltd: Current Employment. Barlera:Member of the DSMB for an independent (not sponsored) study conducted at the Mario Negri Institute, no fees: Other. Hatzi:Genentech, Inc., F. Hoffmann-La Roche Ltd: Current Employment, Current equity holder in publicly-traded company. Jiang:F. Hoffmann-La Roche Ltd: Current Employment, Current equity holder in publicly-traded company. Boyer:F. Hoffmann-La Roche Ltd: Current Employment. Morschhauser:F. Hoffmann-La Roche Ltd, Gilead, AbbVie: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd, AbbVie, BMS, Genmab, Gilead, Novartis: Consultancy.
Venetoclax plus Pola-R-CHP is an investigational combination. Venetoclax (Venclexta) is a BCL-2 inhibitor indicated: for the treatment of adult pts with CLL or SLL; in combination with azacitidine, or decitabine, or low-dose cytarabine for the treatment of newly diagnosed AML in adults 75 years or older, or who have comorbidities that preclude use of intensive induction chemo. Polatuzumab vedotin (Pola) is a CD79b-directed antibody-drug conjugate indicated: in combination with a rituximab product, cyclophosphamide, doxorubicin, and prednisone (R-CHP) for the treatment of adult patients who have previously untreated DLBCL, NOS or HGBL and who have an IPI score of 2 or greater; and in combination with bendamustine and a rituximab product for the treatment of adult pts with relapsed or refractory DLBCL, NOS after at least two prior therapies.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal